axumin pet scan indications
The Axumin images were originally read by on-site readers. You will be asked to lie still on the exam table for 20 30 minutes while the scanner captures images of the area s being investigated.
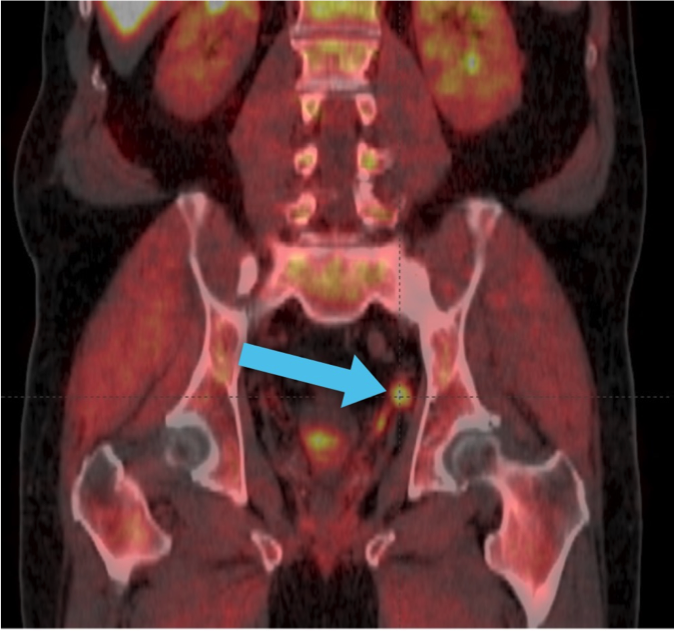
Axumin Fluciclovine F 18 Injection Case Studies For Axumin
The painless scan takes about 40-45 minutes.
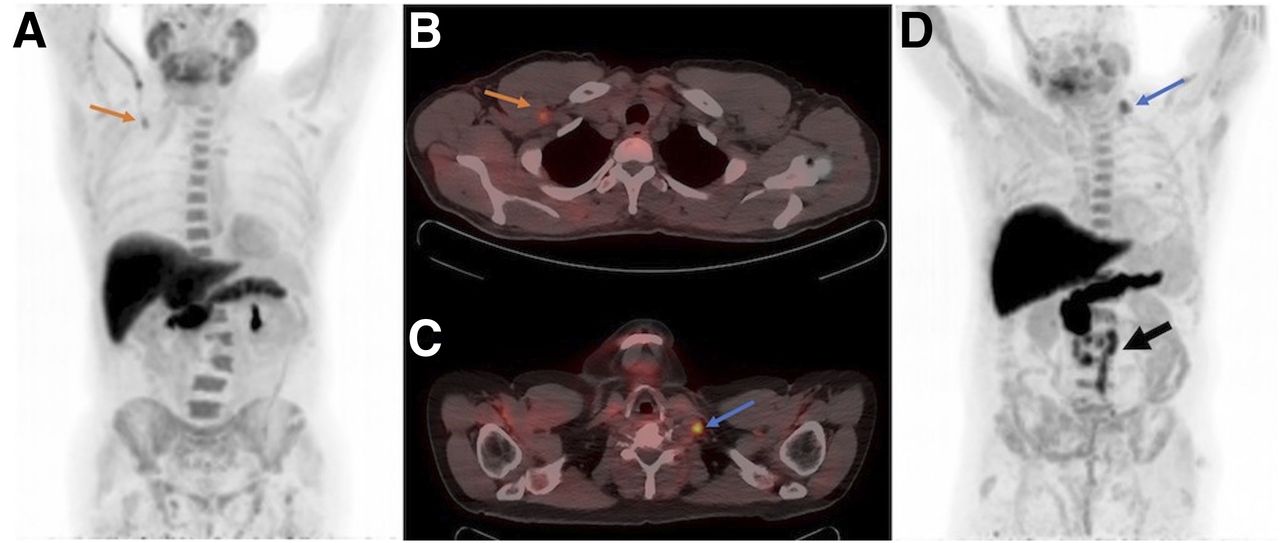
. Prior to Scan. PET-CT can also be used as a problem-solving tool for example. Axumin fluciclovine F 18 injection is indicated for positron emission tomography PET in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen PSA levels following prior treatment.
Lymphoma testicular seminoma 4. Amino acids are the building. Home axumin pet scan wallpaper.
The University of Tennessee Medical Center has been offering Axumin scans. INDICATIONS AND USAGE. The NCCN Clinical Practice Guidelines in Oncology NCCN Guidelines for Prostate Cancer Version v22022 state that F 18 fluciclovine PETCT or PETMRI should be considered as options in the clinical workup of patients with recurrence or progression of nonmetastatic prostate cancer.
A 18 F-Fluciclovine Axumin is a nuclear medicine study using positron emission tomography PET with computed tomography CT to localize recurrent disease in patients who were previously treated for prostate cancer and are now presenting with an increased prostate-specific antigen PSA. 78815 PETCT skull base to mid-thigh. Axumin pet scan indications Wednesday May 18 2022 Edit.
When CT is complete approximately 25 secs you will be prompted to move patient for PET scan table moves all the way to the back of the gantry 6. In brief the Axumin PETCT scan generic name 18F-fluciclovine is a total body metabolic scan identifying tumors based on their proliferation rates. Currently the FDA approved indication applies to cases of PSA relapse suspected recurrent disease after primary therapy.
Medicare Oncologic PET Reimbursement Guidelines. In the absence of an NCD coverage determinations for all oncologic and non-oncologic uses of PET that are not included in another NCD under section 2206 will be made by the Medicare Administrative Contractors under section 1862 a 1 A of the Social Security Act. All PET indications currently covered or non-covered under NCDs under.
Set parameters for scan 4. Study 1 evaluated 105 Axumin scans in comparison to histopathology obtained by biopsy of the prostate bed and biopsies of lesions suspicious by imaging. If the PSA is less than 1 the detection rate is 375.
After the exam you can return to your normal diet and activities. The indications for F-18 fluorodeoxyglucose FDG PET-CT imaging include. Axumin is a diagnostic medicine used with a body scan to check whether or not prostate cancer has returned.
Axumin is indicated for positron emission tomography PET in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen PSA levels following. Axumin has been FDA approved since 2016 but for only for restaging of prostate cancer. The highly sensitive PET scan detects the metabolic signal of actively growing cancer cells in the body and the CT scan provides a detailed picture of the internal anatomy that reveals the location size and shape of abnormal.
Options PET monitor to view scan length if desired applies to UNMH. The National Comprehensive Cancer Network guidelines NCCN Version 4. PET-CT continued Page 3 of 3 position.
PSMA PET scans are FDA approved for -both- initial staging of prostate cancer for men recently diagnosed with certain types of higher risk prostate cancers as well as for restaging of men with suspected recurrent prostate cancer. Allow approximately 1 hour for the entire Axumin PETCT study. The results of a US cost-consequence study Jensen et al.
Since PET and CT technology each have some medical limitations we perform hybrid exams during a single session eliminating the need for separate scans. 2017 published as an abstract only suggests that increased use of the technology for suspected prostate cancer recurrence may lead to better clinical outcomes while being cost. Indication CPT Coverage Guidelines Suspected recurrent prostate cancer ICD-10 78815 C61.
The company also claims that Axumin use has the potential to reduce the pre-patient scan time compared to alternative PET tracers. The Axumin radiotracer will be injected into your arm via an IV. Axumin fluciclovine F 18 is a radioactive tracer approved by the FDA in 2016 for positron emission tomography PET imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen PSA levels following prior treatment.
Allow 15 minutes for interview IV injection Image acquisition. The most common side effects of Axumin include injection site pain injection site erythema and dysgeusia. 2 DOSAGE AND ADMINISTRATION.
Evaluation of suspected disease recurrence relapse andor residual disease eg. Axumin may not accumulate in densely sclerotic bone metastases so careful review of the CT. 18 F-Fluciclovine is a synthetic amino acid analog.
Axumin Imaging Interpretation Manual Axumin Imaging Interpretation Training Manual v20 FINAL 05Jun19 4 of 21 3 Indication and Important Safety Information 31 Indication Axumin fluciclovine F18 injection is a radioactive diagnostic agent indicated for positron emission tomography PET imaging in men with suspected prostate cancer recurrence based. To view the full NCCN Guidelines for Prostate Cancer. PETCT imaging generally included the abdomen and pelvic regions.
If the PSA is above 2ngml the detection rate of tumor recurrence is 917. Axumin PET CT is less sensitive for recurrent tumor detection in patients with a PSA level below 2ngml. Occult primary lesion eg.
Load move start 5. F-18 Fluciclovine Axumin PET-CT Clinical. The images were subsequently read by three blinded independent readers.
Non-metastatic manifestation of neoplastic disease. It is used specifically with the body scan known as positron-emission tomography PET in men whose blood test for prostate-specific antigen PSA indicate that the cancer may have returned. 21 Radiation Safety -.
Axumin is indicated for positron emission tomography PET in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen PSA levels following prior treatment. Axumin is a radiopharmaceutical.
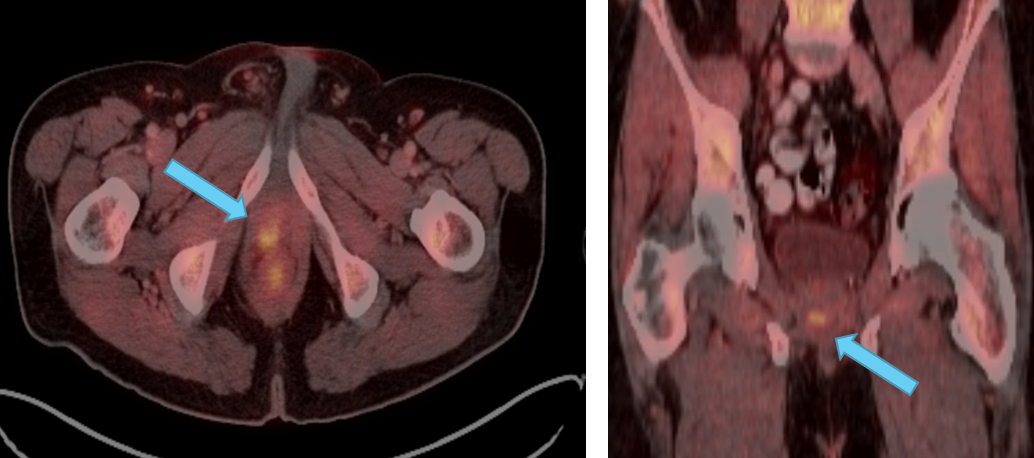
Axumin Fluciclovine F 18 Injection Case Studies For Axumin
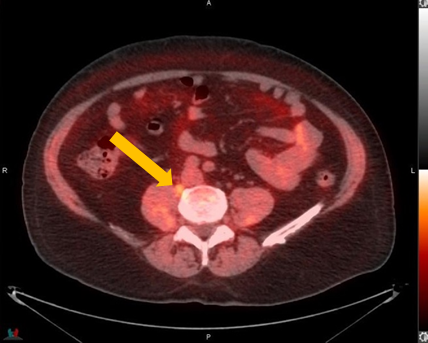
Axumin Fluciclovine F 18 Injection Case Studies For Axumin

Understanding The Clinical Impact Of Utilizing Axumin 18fluciclovine Dms Health

Understanding The Clinical Impact Of Utilizing Axumin 18fluciclovine Dms Health
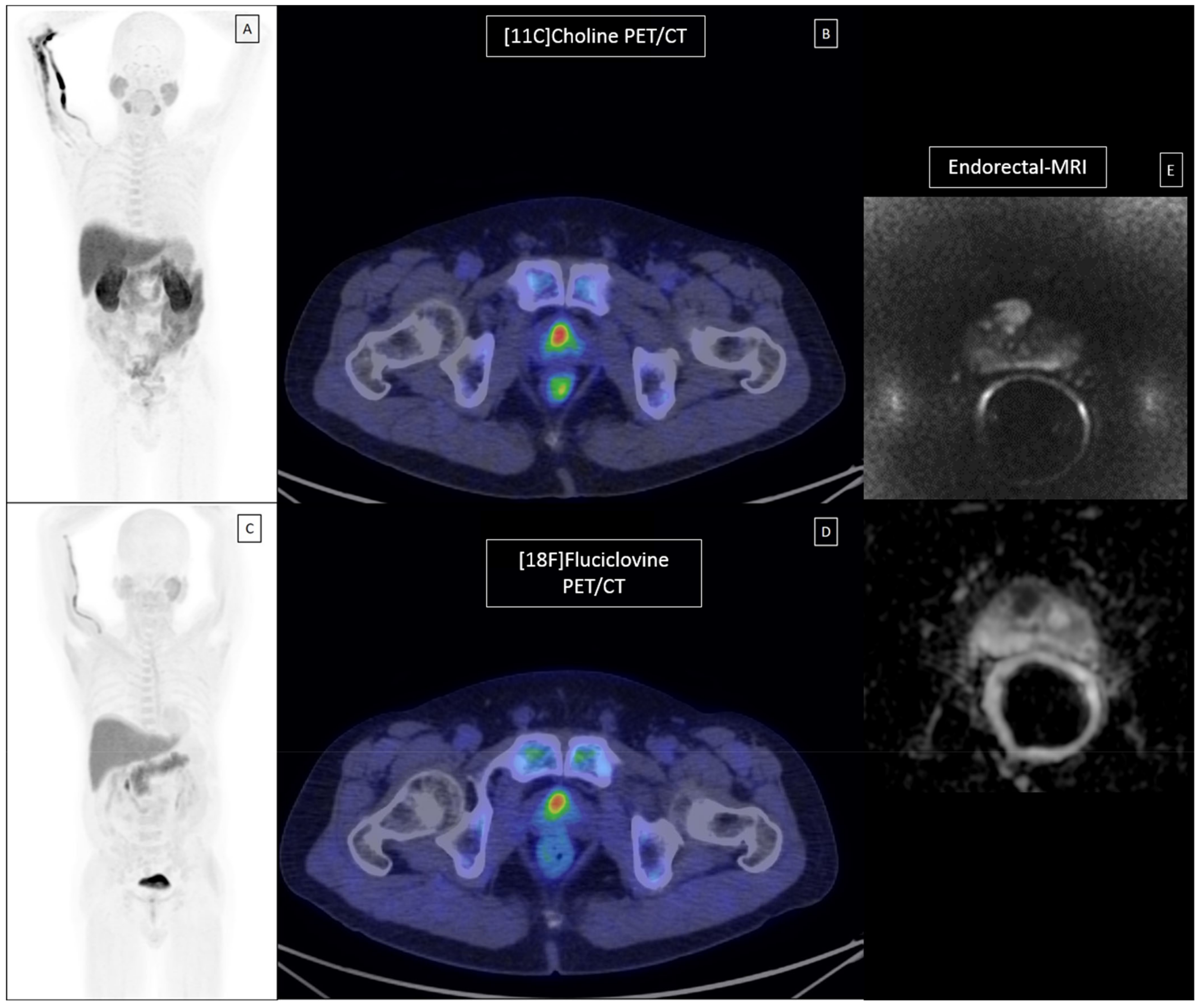
Cancers Free Full Text The Role Of 18f Fluciclovine Pet Ct In The Characterization Of High Risk Primary Prostate Cancer Comparison With 11c Choline Pet Ct And Histopathological Analysis Html

Best Practices For 18f Fluciclovine Pet Ct Imaging Of Recurrent Prostate Cancer A Guide For Technologists Journal Of Nuclear Medicine Technology
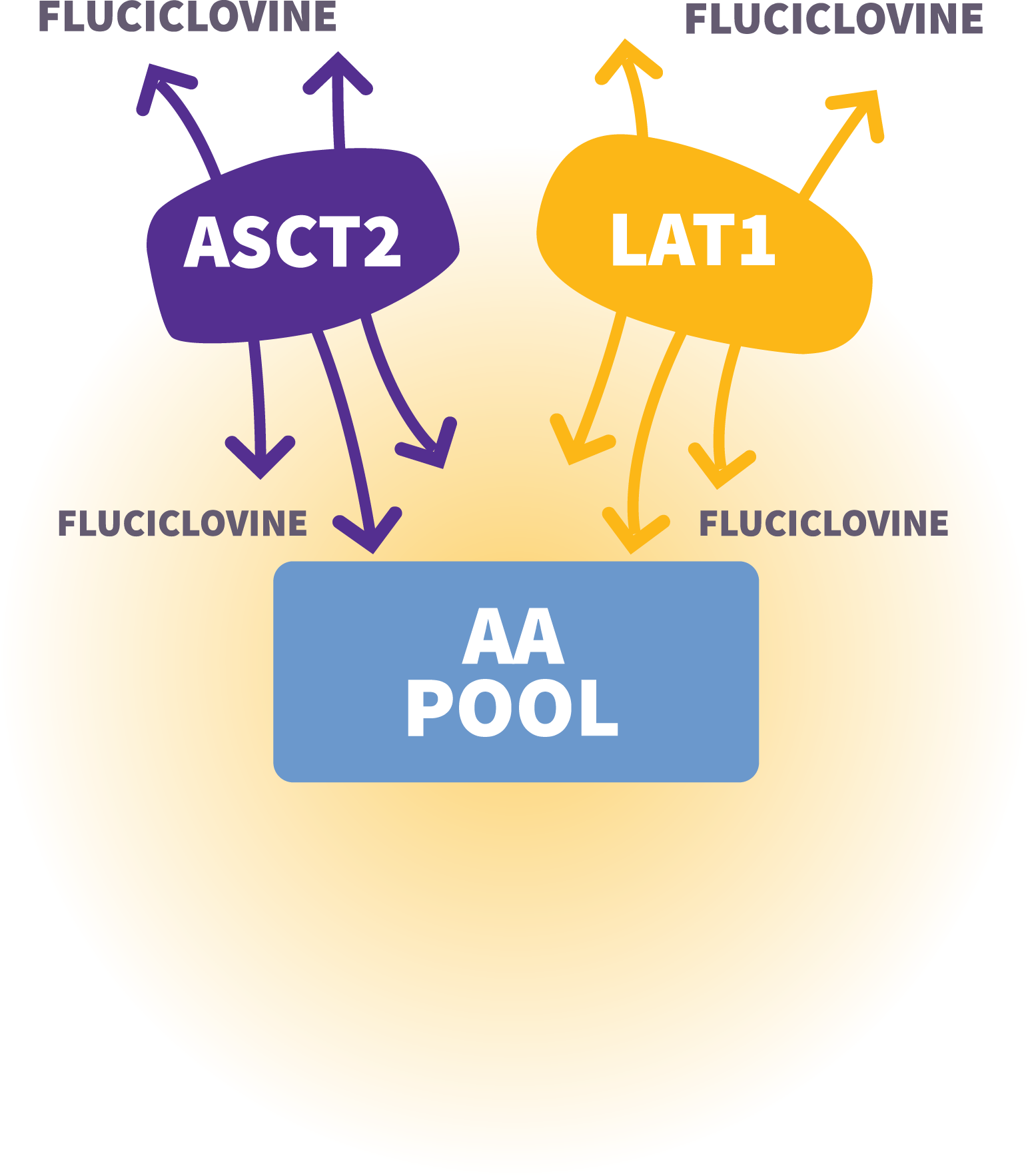
Axumin Fluciclovine F 18 Injection Mechanism Of Action For Axumin
Results Published For Axumin Fluciclovine F 18 Pet Imaging Study Demonstrating Improved Patient Outcomes In Patients With Recurrent Prostate Cancer Imaging Technology News
Blue Earth S Phase 3 Axumin Imaging Study Shows Promise In Glioma Pet Scans Fierce Biotech

Axumin Pet Agent Added To Nccn Guidelines For Suspected Recurrent Prostate Cancer Imaging Technology News
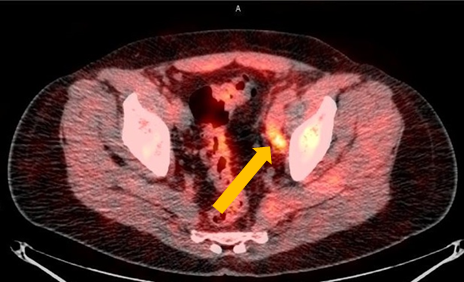
Axumin Fluciclovine F 18 Injection Case Studies For Axumin
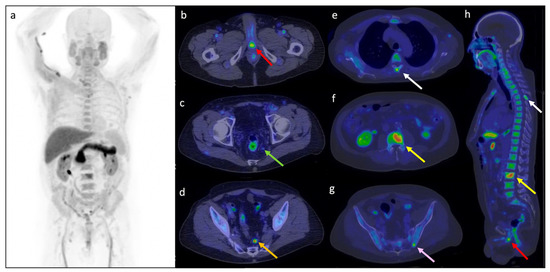
Cancers Free Full Text 18f Fluciclovine Pet Ct Improves The Clinical Management Of Early Recurrence Prostate Cancer Patients Html
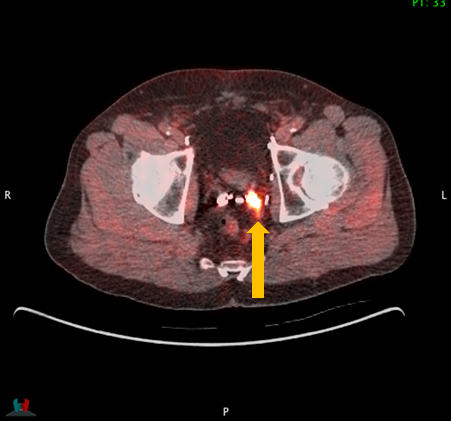
Axumin Fluciclovine F 18 Injection Case Studies For Axumin

Best Practices For 18f Fluciclovine Pet Ct Imaging Of Recurrent Prostate Cancer A Guide For Technologists Journal Of Nuclear Medicine Technology

Best Practices For 18f Fluciclovine Pet Ct Imaging Of Recurrent Prostate Cancer A Guide For Technologists Journal Of Nuclear Medicine Technology